US FDA approves Shorla’s oncology drug for T-cell leukaemia
Pharmaceutical Technology
MARCH 8, 2023
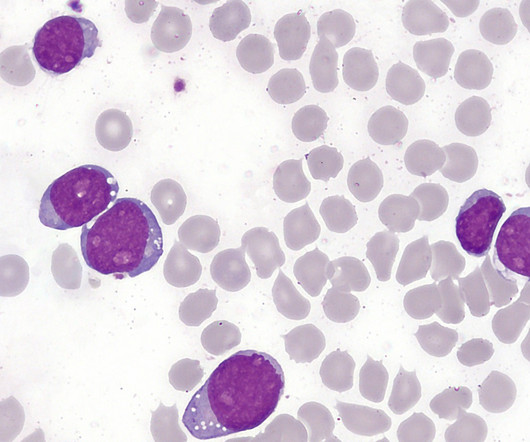
The US Food and Drug Administration (FDA) has granted approval for Shorla Oncology’s Nelarabine Injection, an oncology drug, to treat T-cell leukaemia, an aggressive blood and bone marrow cancer that progresses quickly. The regulatory approval marks the company’s first product to receive approval in the US market.






































Let's personalize your content